- Your cart is empty
- Continue Shopping
- Home /
- Page
Fenbendazole 444 Mg Australia
Fenbendazole Tablets For Humans
$80.00 – $280.00Fenbendazole 222Mg
$70.00 – $260.00Fenbendazole 444Mg
$75.00 – $270.00Fenbendazole 150Mg
$68.00 – $250.00Mebendazole 100mg (Mebex)
$35.00 – $120.00Albendazole 400 mg USA, UK, AUS
$80.00 – $2,550.00What is Fenbendazole 444 mg? A New Look at an Old Antiparasitic
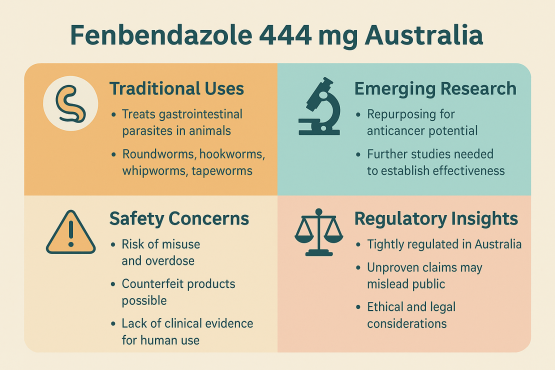
Fenbendazole is a benzimidazole anthelmintic widely used to treat gastrointestinal parasites in animals. The “Fenbendazole 444 mg Australia” formulation refers to a high‐strength capsule variant being offered in Australia, often for veterinary or experimental use. This strength is above many standard doses, so it demands careful attention to safety, regulation, and novel potentials beyond its traditional uses. Fenbendazole Australia is increasingly recognized not only for its 444 mg strength in effective parasite control but also for its potential role in new areas of medical and veterinary research, attracting attention from both professionals and curious readers.
Traditional Uses & Mechanism of Action
How Fenbendazole Works
It binds tubulin in parasitic organisms, disrupting formation of microtubules which are essential for their nutrient absorption and reproduction. This leads to starvation and death of the parasites.
Used in livestock, dogs, cats, sheep etc., against roundworms, hookworms, whipworms, lungworms, certain tapeworms.
Dosage and Variations
Standard veterinary doses often involve lower mg amounts per body weight over multiple days.
There are different strengths globally. For instance, Fenbendazole 222 mg Australia appears in some listings, representing a mid‐range dosage. Careful attention to labels, species and weight is essential.
Emerging Uses & Research — What’s New
Repurposing for Anticancer Research
Some preclinical studies suggest that fenbendazole may inhibit cancer cell growth in vitro. For example, research using mouse tumor cells (EMT6 mammary tumor cells) has shown cytotoxic activity under certain conditions.
Another study combining fenbendazole with nutritional/vitamin supplements showed tumor suppression in animal models.
However, no rigorous human clinical trial has yet confirmed effectiveness or safety for these uses.
Regulatory & Legal Roadblocks in Australia
Therapeutic Goods Administration (TGA) regulates all therapeutic and veterinary chemicals. Products must be approved or listed to be legal for human or veterinary medical use. Unregistered use can be illegal.
For livestock (goats, sheep etc.), even worm drenches must often be prescribed “off‐label” by a veterinarian if the product isn’t specifically registered for that animal. Dosage differences and withdrawal periods are governed strictly.
Safety, Side Effects & Ethical Considerations
Known Safety Profile
Among animals, fenbendazole has a relatively good safety margin. Many species tolerate it well; side effects are typically mild (digestive upset, transient appetite change).
Toxicology data indicate low acute toxicity; strong exposure (very high doses) seldom but possibly harmful.
Risks in High‐Strength Use
With high mg formulations such as 444 mg, risk of misuse increases (overdose, off‐label human use, inadequate veterinary supervision).
There is concern about counterfeit or unverified purity products when sourcing via non‐regulated channels.
Ethical & Regulatory Concerns
Promoting fenbendazole for human disease treatment without strong clinical evidence can mislead vulnerable people.
Authorities like APVMA (Australia’s version) and TGA monitor claims and registration status. Importing therapeutic goods unregistered for a given use may violate law.
Distinctive Insights — What Most Blogs Don’t Cover
Pharmacokinetics in Different Species & Environments
Absorption, metabolism, excretion differ markedly: e.g., ruminants (sheep, goats) break down drugs differently in rumen; so drug half‐life and effective dose may vary.
Environmental factors (temperature, diet, breed) can affect how well fenbendazole penetrates parasite tissues.
Resistance Risks & Sustainability
With frequent use of high doses, parasites may evolve resistance. Monitoring is sparse in many regions. Encouraging rotating drugs or combining anti‐parasitic strategies can delay resistance.
Sustainable dosing: ensuring withdrawal periods in food animals (milk, meat) are respected to avoid residues.
Quality Control & Purity Verification
Capsules of 444 mg strength should ideally come with certificates of analysis (COA) / third‐party lab testing.
Ingredient sourcing, manufacturing conditions (contamination, uniformity) matter especially in high‐potency products.
Practical Advice for Users & Stakeholders
For Veterinarians & Farmers
Consult regulatory status of the specific product you intend to use; always follow label instructions where available.
When using off‐label or experimental high doses, document everything — dose, animal weight, withdrawal period.
For Regulators & Policy Makers
Promote transparency: enforce requirements for purity testing, proper labeling.
Consider funding or facilitating trials on high‐strength vs standard doses in animals/humans (where ethical) to establish safety & efficacy.
For Consumers & Pet Owners
Do not self‐administer fenbendazole (especially formulations labelled for animals) for human health conditions.
Seek veterinary or medical advice before using, and verify source legitimacy.
Conclusion
“Fenbendazole 444 mg Australia” represents more than just a high‐strength veterinary antiparasitic. It lies at the intersection of regulatory oversight, research innovation, risk and potential. The future may hold repurposed roles (e.g. in disease beyond parasites), but for now safe use, compliance, purity, and evidence are paramount. Stay informed, demand quality, respect the law—and let science guide potential new uses, rather than hype.






